Jessenia Cornejo, Senior Consultant of Compliance and Quality, will be speaking at HCCA Compliance Institute on April 24, 2023.
The title of the talk:
How to Develop an Effective Risk Management Program

Jessenia Cornejo, will be speaking at HCCA Hawaii Regional Healthcare Compliance Conference on October 7, 2021.
Jessenia Cornejo, Senior Consultant of Compliance and Quality, will be speaking at HCCA Hawaii Regional Healthcare Compliance Conference on October 7, 2021. The conference is available for online attendance.
The title of the talk:
Maximizing Your Audit & Monitoring Program
Find out more at the HCCA website.
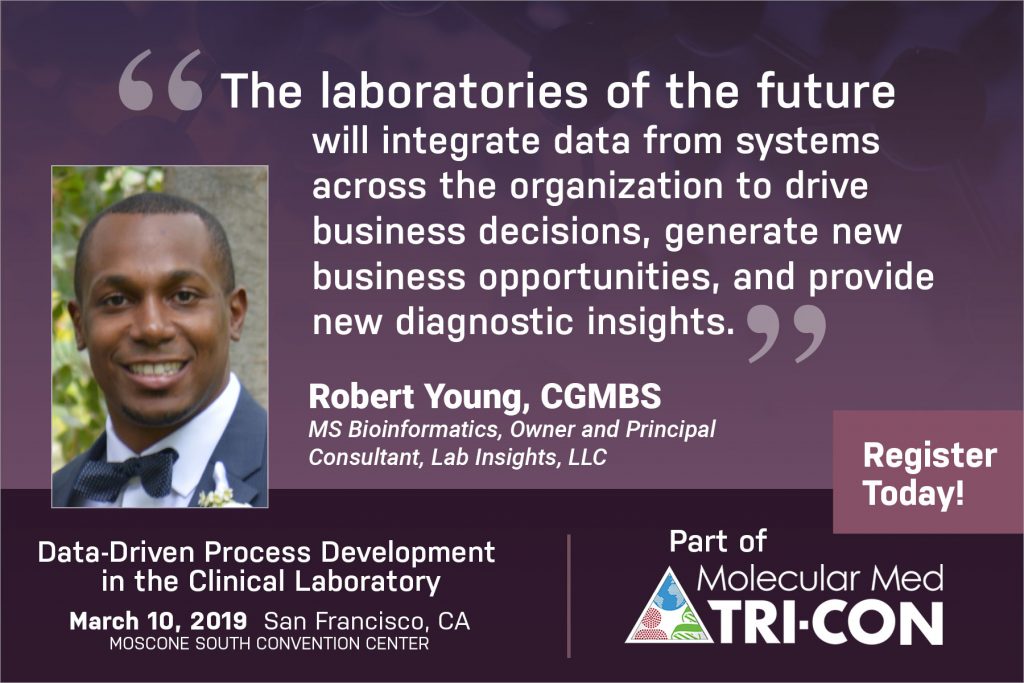
Lab Insights Founder, Robert Young, is a confirmed speaker at the 11th annual Next Generation Dx Summit. He will be leading a short course titled Data-Driven Process Development in the Clinical Laboratory. The conference will take place in Washington D.C. from August 20-22 2019. The course is scheduled for the 21st at 6:45 pm.

Lab Insights Founder, Robert Young, is a confirmed speaker at the 11th annual Next Generation Dx Summit. He will be leading a short course titled Data-Driven Process Development in the Clinical Laboratory. The conference will take place in Washington D.C. from August 20-22 2019. The course is scheduled for the 21st at 6:45 pm.
The modern clinical laboratory utilizes complex, data-rich computer systems. In this course, you will learn how to leverage your laboratory information systems to build data-driven processes, create data-driven process improvements, and make data-driven business decisions.
Short course tagline
The course is intended for laboratory and health IT professionals who want to learn more about how to build data-centric processes and electronic systems in the age of machine learning. To find out more about the conference, click here.
The modern clinical laboratory utilizes complex, data-rich computer systems. In this course, you will learn how to leverage your laboratory information systems to build data-driven processes, create data-driven process improvements, and make data-driven business decisions.Key topics that were covered:
On Friday, November 18th, the Food and Drug Administration (FDA) announced that it would not seek to finalize its guidance on laboratory developed tests (LDTs) prior to the Trump Administration taking office. Since then, the agency has elaborated on some of the details of this announcement, claiming:
While excessive oversight can discourage innovation, inadequate and inconsistent oversight in which different test developers are treated differently can also discourage innovation by making it difficult for high-quality test developers to compete with poorer performing counterparts.
In an effort to protect the site, and in a broader effort to keep the Internet safe, labinsightsllc.com has gone to https.
To find out more, visit letsencrypt.org
Coming Soon…
In February 2014, the FDA submitted its final rule regarding updates to 21 CFR 803, Mandatory Reporting Requirements: Manufacturers, Importers and Device User Facilities. Up until now, manufactures and importers were able to submit a paper form, known as 3500A form, or an electronic equivalent. Effective August 1 2015, electronic submissions will be the only acceptable format. These submissions must be in HL7 format and sent to the FDA’s Electronic Submissions Gateway. This will be a drastic change in the way MDR submissions occur for many organizations. Luckily, the Administration has created a free desktop application that manufactures and importers can use to generate the HL7 files. In addition, the FDA has created an API for organizations to use to turn their existing complaints management solutions into the HL7 format. For more information, read on the FDA’s website here and here.
NO EVENT SHALL LAB INSIGHTS, LLC BE LIABLE, WHETHER IN CONTRACT, TORT, WARRANT, OR UNDER ANY STATUTE OR ON ANY OTHER BASIS FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE OR CONSEQUENTIAL DAMAGES IN CONNECTION OR ARISING FROM LAB INSIGHTS, LLC SERVICES OR USE OF THIS DOCUMENT.
Originally written March 20 2014
Changes to both CLIA and HIPAA regulations now allow for patients to directly access results from the performing laboratory. Previously only, “authorized persons” such as the ordering physician or laboratory were able to access lab results, requiring patients to have to go through them to received their own data. This new development is consistent with new directives in healthcare to allow patients “to take a more active role in managing their [own] health and health care.” Click here for the final rule itself.
NO EVENT SHALL LAB INSIGHTS, LLC BE LIABLE, WHETHER IN CONTRACT, TORT, WARRANT, OR UNDER ANY STATUTE OR ON ANY OTHER BASIS FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE OR CONSEQUENTIAL DAMAGES IN CONNECTION OR ARISING FROM LAB INSIGHTS, LLC SERVICES OR USE OF THIS DOCUMENT.